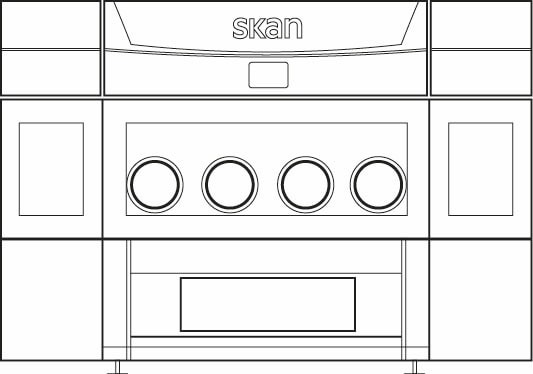
SKAN pure² Isolator - 4-glove working chamber
Product code : SKA.1391030
SKA.1391030The SKAN pure² isolator is built, tested and certified according to the standards and regulations for isolators for the production of cytostatics and for closed microbiological workbenches.

Via de knop “offerte aanvraag” kan u vrijblijvend contact met ons opnemen voor verdere informatie.
SKAN pure² Isolator - 4-glove working chamber
Product beschrijving
Requirements
- Safe containment that satisfies all cleanroomrequirements (cGMP class A/ISO class 5 in accordance with ISO 14644)
- Wide range of applications
- Fast and automated H2O2 decontamination
- Excellent ergonomics and working conditions combined with maximum safety
- Compliance with applicable regulatory and standardised requirements
Standards & Certifications
In accordance with Machinery Directive 2006/42/EC, EMC Directive 2014/30/EC, DIN EN 12469, DIN 12980, ISO 14644-7, cTUVus certified
Solution
- SKAN pure²: An aseptic and aseptic-toxic working isolator
- Closed containment provides a safe handling condition also when working with high potent products
- Fast and safe H2O2 decontamination cycles with the patented skanfog technology
- Large and fast airlock to improve isolator productivity
- No connection to HVAC required due to integrated SKAN nanox catalyst system
Functional principle
SKAN combines extensive knowledge of laboratory workbenches and isolator technoloy. The SKAN pure² isolator is built, tested and certified according to the standards and regulations for isolators for the production of cytostatics and for closed microbiological workbenches.
Characteristics
- Working chamber and airlock in stainless steel
- Smooth and easy to clean working surface
- Selectable positive or negative pressure operation
- Maintains GMP cleanroom Class A, ISO 5 standards
- User-friendly Siemens P7-1500 with 9“ color touch screen control panel
- Electronic batch recording
- All working and maintenance openings are accessible from the front, ideal for wall installation
- Easy assembly, installation and access through standard doorways and elevators
Field of application
- Cell & Gene:
- Cell therapy
- Cell cultures
- Transformed DNA
- Sterility testing:
- Quality control
- Pharmaceutical manufacture
- Carcinogenic, mutagenic or reprotoxic substances (CMR)
- Total parenteral nutrition (TPN)
- Intravenous solution (IV)
- Cytotoxic
- Virostatic
- Anti-neoplastic chemotherapy
- Transformed DNA
- Small scale aseptic pharma production
- Biosafety:
- Pathogenic microbiology / viruses
- Transformed DNA
Additional products and options
- Two working chamber sizes available, either with two or four glove ports.
- The airlock with a shelf is available on the right, on the left or on both sides of the isolator.
- SKAN pure² can be equipped with an additional glove testing system.
SKAN pure² Isolator - 4-glove working chamber
Product specificaties
Packaging Unit
1 Unit
Brand
SKAN